22+ Atomic Number Calculator
In order to calculate the average atomic mass of the element we need to multiply the abundance decimal percentage of each isotope by its atomic mass and sum these resulting. How to calculate Atomic Mass using this online calculator.

Periodic Table Of The Elements Math Tutor Dvd Online Math Help Math Homework Help Math Problems Math Practice
So an atom with the.
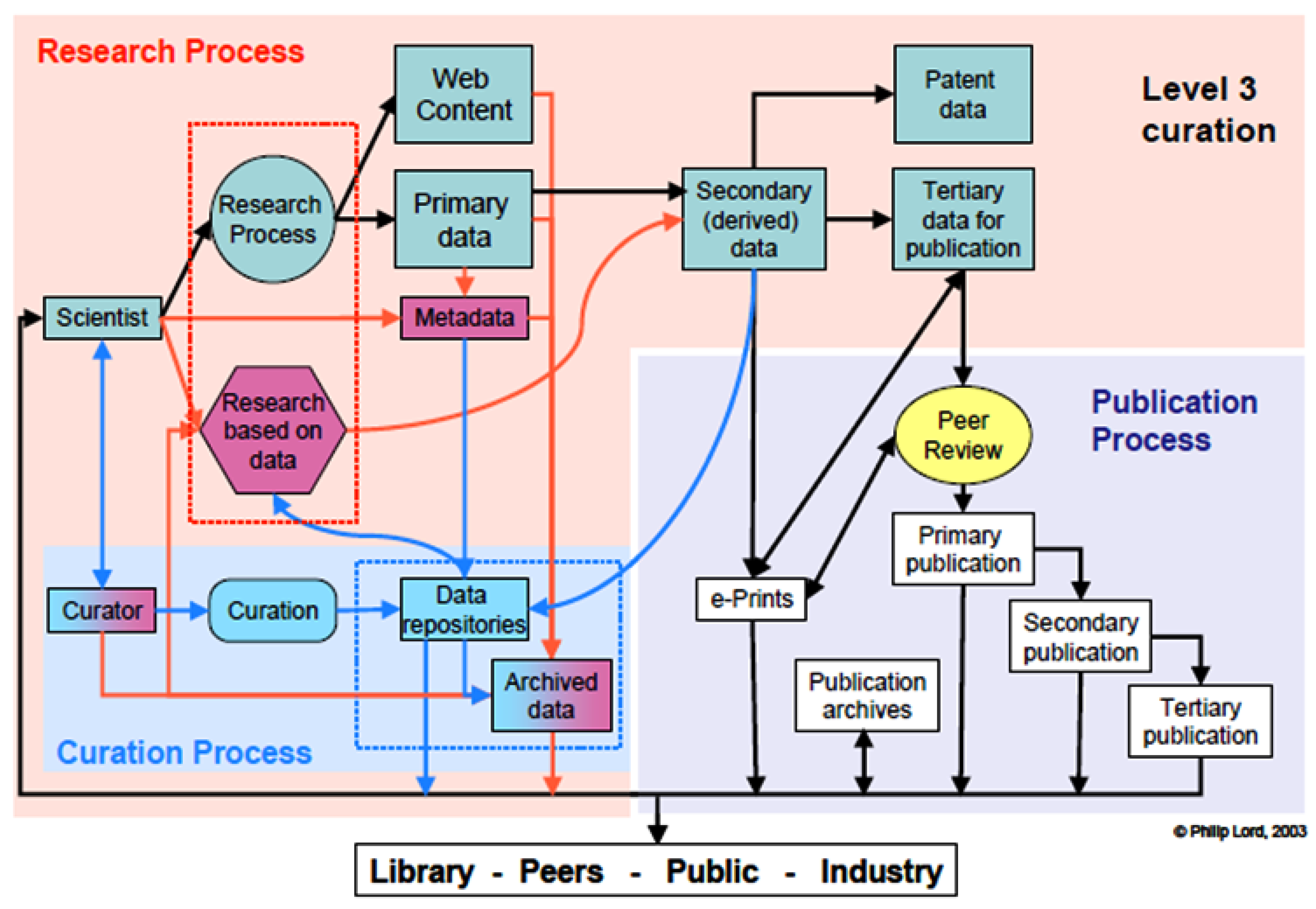
. 23 V Vanadium 509415. Periodic Table Calculator gives the atomic number atomic weight atomic properties nuclear properties electromagnetic properties and others. In related terms another unit of mass often used is Dalton Da or unified atomic mass unit u when describing atomic masses and molecular masses.
The atomic number of an element informs us how many electrons there are in the atoms. Free Chemical Properties calculator - Find symbol atomic number mass boiling point melting point and proton count of atoms. If you enter a value greater than 177 the calculator will warn you because this is the greatest number of neutrons ever recorded in an.
Enter either the Nitrogen or the N symbol in the given empty box of the Valence electron calculator. 25 Mn Manganese 54938044. 26 Fe Iron 55845.
24 Cr Chromium 519961. 27 Co Cobalt. The mass number is a proton number neutron number b electron number proton number c neutron number electron number.
Isotopes are two or more types of atoms that have the same number of. An online atomic weight calculator determines the atomic mass by the following steps. First Enter the number of protons Z and the number of neutrons N.
Lets say you have to calculate valence electrons in nitrogen. You just need to provide the chemical name. Carbon has an atomic number of 6 which translates to 6 electrons as 24.
Yes this free orbital. Atomic Mass Calculator Results detailed calculations and formula below The atomic mass M is kg kilograms Atomic Mass Calculation M Z m p A - Z m n M - M M. Conventional atomic weights are used for.
An online noble gas electron configuration calculator provides a condensed method of finding the electron configuration atomic number and atomic mass of given. To use this online calculator for Atomic Mass enter Total mass of proton mp Total Mass of Neutron mn and hit the. It is known that most chemical elements occur in nature as a mixture of two or more isotopes.
Counting as if you were reading from left to right from top to bottom the position of an element corresponds to their atomic number. 22 Ti Titanium 47867. The periodic table comes in our help.
The formula that is generally used to calculate the atomic mass for an element is as follows Atomic mass number of protons number of neutrons A n Z Here A is the mass. Enter the atomic number of neutrons. Calculate the relative atomic mass of an.
It is defined to be 112 of the mass of.

If 224 Ml Of A Triatomic Gas Has A Mass Of 1 G At 273 K And 1 Atm Pressure Then The Mass Of One Atom Is

The Ean Of Metal Atoms In Fe Co 2 No 2 And Co2 Co 8 Respectively Are

Pdf Calculation The Energy Levels And Energy Bands And Energy Ratios For Even Even154 156 158dy Isotopes Iosr Journals Academia Edu

Atomic Mass Calculator Calculate Neutrons Protons Electrons
:max_bytes(150000):strip_icc()/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)
Molarity Example Problem Converting Mass To Moles

Manual Solution By Nhan Issuu
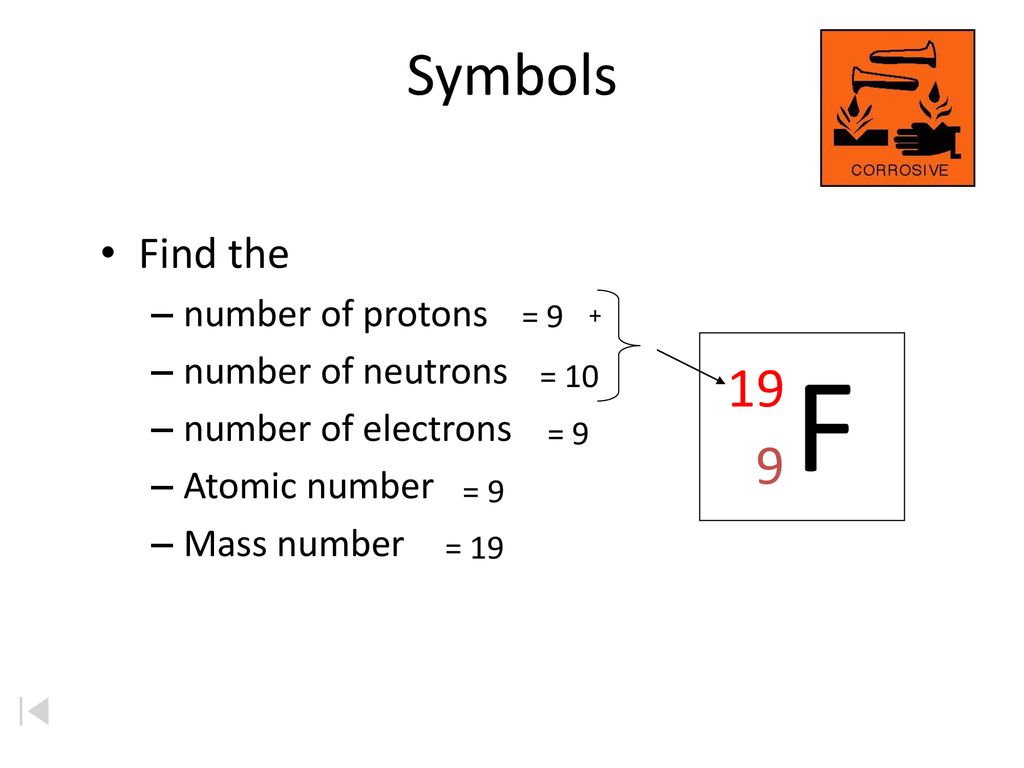
Symbols Contain The Symbol Of The Element The Mass Number And The Atomic Number X Mass Number Atomic Protons Neutrons Mass Number Protons Ppt Download

Browse Questions For Chemistry 101

Trapezoidal Rule For Integration Definition Formula And Examples
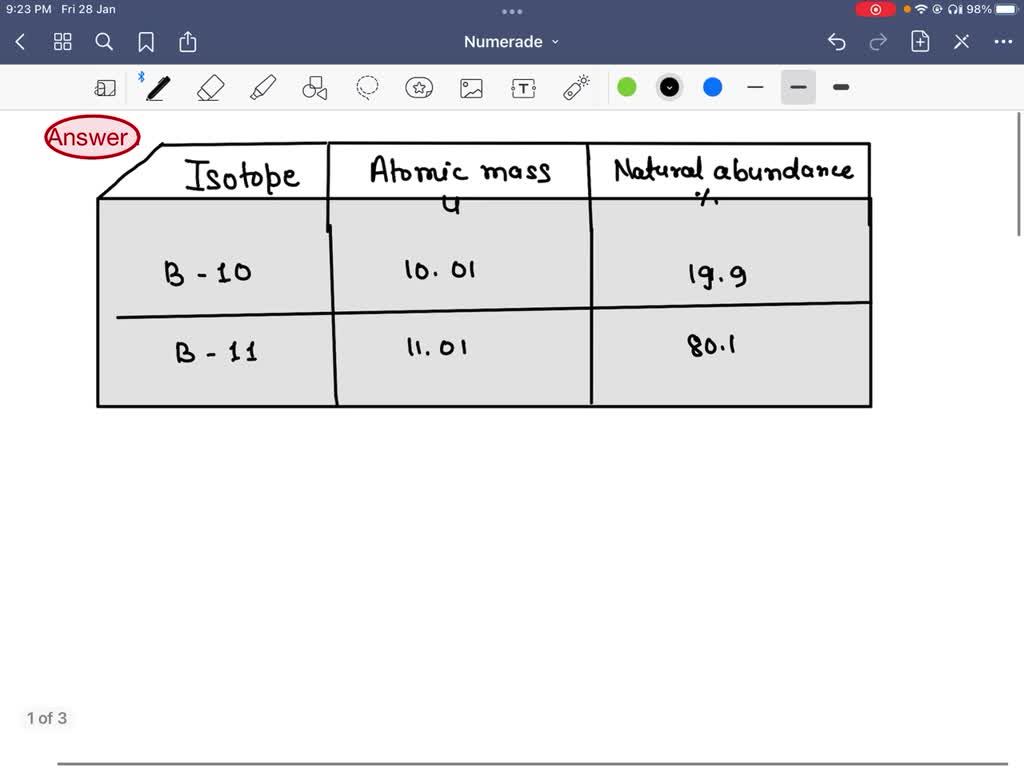
Solved Atomic Structure Coriplete The Followring Chart Pnide Lochon Chene Relative Kass Ftoton Kutoi Deqton Compictc Thc Following Chart Cacmica Symboll Atomkc Number Haat Qumi Protons Eectront Iuluent What Are Isotopes What
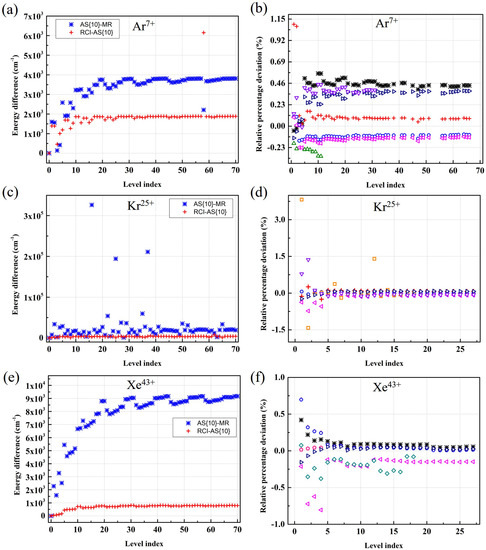
Atoms Free Full Text Extended Calculations Of Atomic Structure Parameters For Na Like Ar Kr And Xe Ions Using Relativistic Mcdhf And Mbpt Methods
First Document Gsi

Class 12 Chemistry Worksheet On Chapter 8 The D F Block Elements Set 4
First Document Gsi

Pdf Calculation Of The Inelastic Longitudinal Form Factors For Some Light Nuclei Fadhil I Sharrad Academia Edu

Properties And Promise Of Catenated Nitrogen Systems As High Energy Density Materials Chemical Reviews

The Charge Exchange Of Slow Highly Charged Ions At Surfaces Unraveled With Freestanding 2d Materials Sciencedirect